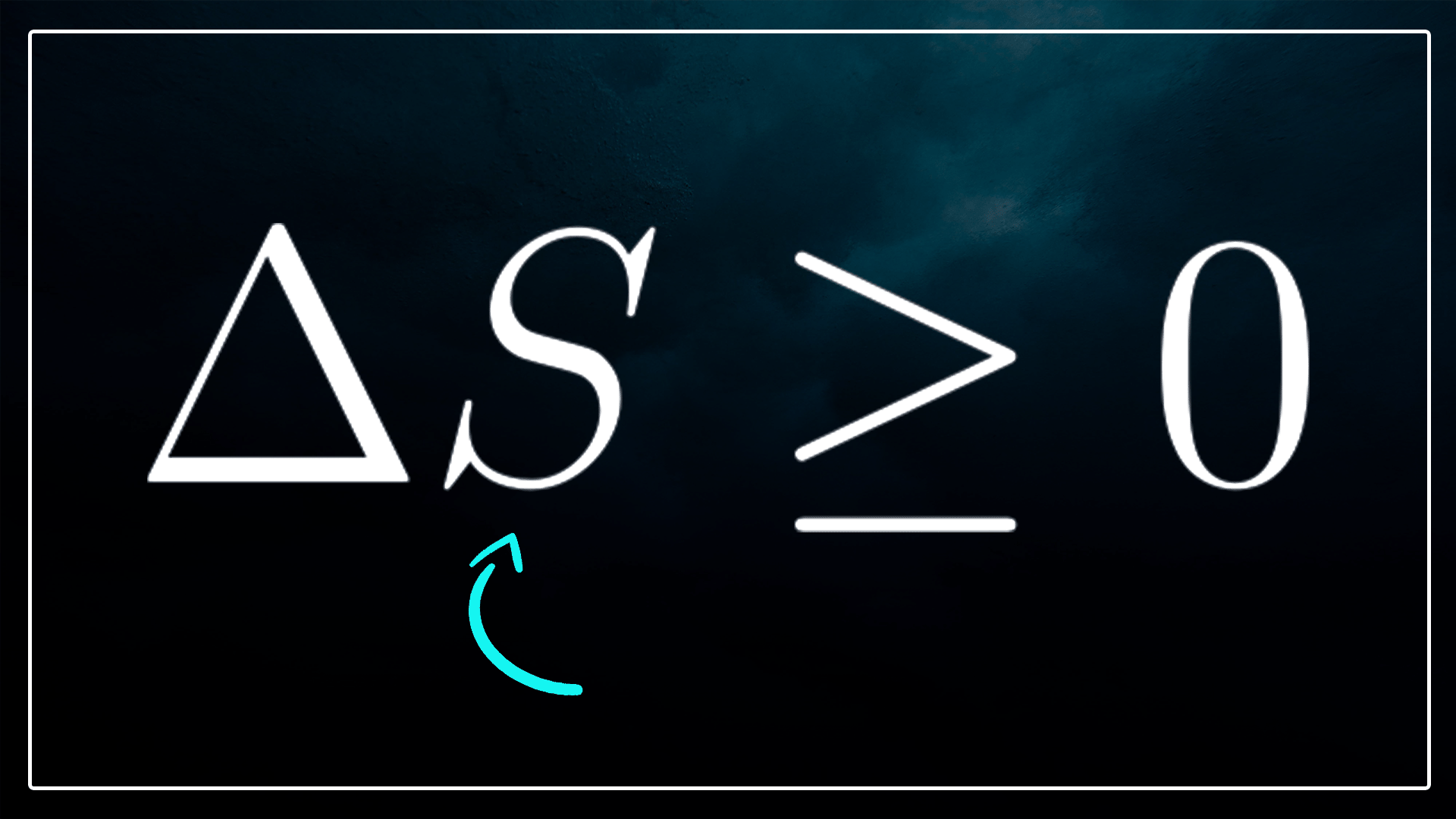The second law of thermodynamics affirms that in any spontaneous process, the entropy of the universe invariably rises. Entropy, in this context, quantifies the degree of disorder or randomness within a system. A system characterized by high entropy boasts an abundance of possible configurations or microstates, while one with low entropy possesses only a limited array of potential configurations or microstates.
Expressed as an inequality, ΔS ≥ 0 dictates that the alteration in entropy for the universe always remains greater than or equal to zero during any process. This principle underscores the impossibility of entropy decreasing in the universe and implies that entropy will ultimately reach its maximum when the system and its surroundings attain thermal equilibrium.
This fundamental law yields several significant implications:
- Spontaneous Heat Flow: Heat cannot naturally transfer from a colder body to a warmer body since such an occurrence would contravene the increase in entropy stipulated by the law.
- Limitations on Heat Engines: The complete conversion of supplied heat into work by a heat engine is unattainable because some heat must be released to the surroundings to augment their entropy. The efficiency of a heat engine is restricted by the Carnot efficiency, which hinges on the temperature differential between the heat source and the heat sink.
- Perpetual Motion Machines: The concept of a perpetual motion machine of the second kind, a device capable of generating work without any energy or matter input, is infeasible. Such a machine would infringe upon the second law of thermodynamics by diminishing the universe's entropy.
If this post was helpful to you, consider subscribing to receive the latest updates! 🙂
And if you would love to see more on physics and engineering topics, please leave a comment under this post!👇
Keep engineering your mind! ❤️
Jousef